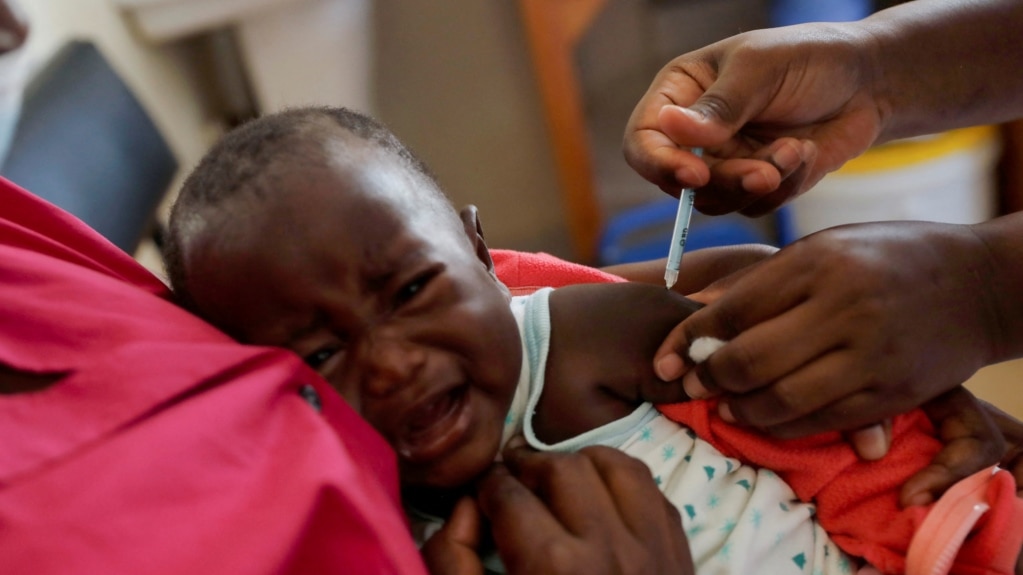
The World Health Organization (WHO) approved a second malaria vaccine last week. The decision could offer a less costly and readily available shot to help fight the disease.
WHO Director-General Tedros Adhanom Ghebreyesus said the U.N. health agency approved the new malaria vaccine based on advice from two expert groups. They suggested the shot could be given to children at risk of the disease.
“As a malaria researcher, I used to dream of the day we would have a safe and effective vaccine against malaria. Now we have two,” Tedros said.
Britain’s Oxford University developed the new three-shot vaccine along with the Serum Institute of India. Research suggests it is more than 75 percent effective. The vaccine can protect people for another year with an additional shot called a booster. Tedros said the shot would cost about $2 to $4 and could be available in some countries next year.
Earlier this year, health officials in the African countries of Ghana and Burkina Faso also approved the vaccine.
Infected mosquitos spread the parasitic disease when they bite people.
“This is one more tool we will now have, but it’s not going to replace bed nets and spraying insecticides,” said John Johnson of the aid group Doctors Without Borders. “This is not the vaccine that’s going to stop malaria,” he added. Johnson was not part of the expert groups that advised the WHO.
The WHO approved the first malaria vaccine in 2021. The U.N. agency described the vaccine development as an “historic” effort to end the deadly disease in Africa. The continent is home to most of the world’s estimated 200 million malaria cases and 400,000 malaria deaths.
But that vaccine, known as Mosquirix and made by GSK, is only about 30 percent effective. The treatment requires four shots and becomes less effective within months.
WHO experts, however, said the data to date on the GSK and Oxford-developed vaccines does not show which one is more effective.
The Bill & Melinda Gates Foundation had been one of the GSK vaccine’s biggest financial supporters. But, the non-profit ended its support for Mosquirix last year. It said the GSK shot was less effective than officials would like and that money would be better used elsewhere.
“The big difference with these two vaccines is access,” Johnson told the Associated Press. He noted that about 10 countries or more could get the GSK vaccine in the next few years.
GSK has said it can only produce about 15 million treatments a year. The Serum Institute has said it could make up to 200 million treatments of the Oxford vaccine a year.
Alister Craig is with Britain’s Liverpool School of Tropical Medicine. He said countries still waiting for the GSK vaccine should turn to the Oxford vaccine instead.
If the new vaccine is widely available across Africa, it could greatly reduce severe sickness and deaths caused by malaria in a few years, Craig said.
Neither of the vaccines stops transmission of malaria. As a result, immunization campaigns alone would not be enough to prevent epidemics. And, reports of resistance to malaria drugs are on the rise.
“You would be foolish to think that this vaccine is going to be the end of the malaria story,” Craig said.
Dengue vaccine
In a separate decision, the WHO’s expert group also approved the dengue vaccine made by Takeda. European Union drug officials approved the dengue vaccine earlier.
The WHO advised that the dengue vaccine be used in children aged 6 to 16 in countries where the disease is widespread.
Dengue killed almost 1,000 people this year in Bangladesh.
I’m Caty Weaver.